The realm of genomic research is rapidly evolving, and innovative technologies are at the forefront of this transformation. One such advancement is the application of high-throughput CUTseq (Circularized Uniquely Targeted sequencing) for single-cell analysis.
This cutting-edge technique has been described in High clonal diversity and spatial genetic admixture in early prostate cancer and surrounding normal tissue by our customers from the Karolinska Institute's Department of Microbiology, Tumor and Cell Biology, not once, but twice (Fig. 1), and is significantly enhanced by the use of the I.DOT Liquid Handler, a tool that promises precision, efficiency, and scalability for cancer research.

Figure 1. High clonal diversity and spatial genetic admixture in early prostate cancer and surrounding normal tissue published in Nature Communications.

Understanding CUTseq
CUTseq is a robust method used to analyze DNA at a single-cell level, providing insights into genomic variations, mutations, and chromosomal alterations. This technology is pivotal in understanding complex biological processes and diseases, including cancer.
In this study, the I.DOT Non-Contact Dispenser was instrumental in processing skeletal muscle tissue samples for single-cell CUTseq analysis.
Application for Genomic Cancer Research
Zhang, N., Harbers, L., Simonetti, M. et al. (2024) performed CUTseq on the MALBAC products largely following the high-throughput CUTseq protocol utilizing the I.DOT for all dispense steps. For the digestion step, they prepared a digestion mix containing 100 nL of NlaIII restriction enzyme and 50 nL of CutSmart buffer per cell. They then dispensed 150 nL of the digestion mix into each well and incubated the plates at 37 °C for 30 min followed by 65 °C for 20 min to inactivate the enzyme.
After digestion, the researchers dispensed 300 nL of 33 nM scCUTseq adapters (prepared as described above) into each well (one barcode per cell), followed by 700 nL of a ligation mix containing 200 nL of T4 rapid DNA ligas , 300 nL of T4 ligase buffer , 120 nL of 10 mΜ ATP, 30 nL of 50 mg/mL bovine serum albumin , and 50 nL of Nuclease-Free Water. They then incubated the plates at 22 °C for 30 min, after which they dispensed 5 μL of Nuclease-Free Water/33 mM EDTA (for a final concentration of 25 mM) into each well and pooled the contents of all the wells of each plate by placing the plates upside down onto a collection plate covered with parafilm, and centrifuged them at 117 × g for 1 min. They carefully transferred the collected solution into a 5 mL low-binding tube and then removed as much as possible of the Vapor-Lock. They then purified the pooled samples with a 1.2 v/v ratio of the sample and Agencourt Ampure XP bead suspension. After purification, the researchers prepared 250 ng of DNA and sheared it using Covaris ME220 Focused-ultrasonicator with a target peak set at 200 base pairs (bp). To prepare sequencing libraries, they followed the CUTseq protocol from Zhang, X., Garnerone, S., Simonetti, M. et al. (2019), with the following minor modifications: (1) They increased the PCR volume to 200 μL and performed a split PCR reaction in 4-tubes strips; (2) They purified the final library with a 0.8 v/v ratio of sample and Agencourt Ampure XP bead suspension, and eluted the purified library in 50 μL of Nuclease-Free Water.
Briefly, the process involved homogenizing tissue samples, isolating nuclei, and sorting single cells (Fig. 2). The I.DOT's precision in handling these delicate samples ensured the integrity of the nuclei, which is crucial for accurate genomic analysis.
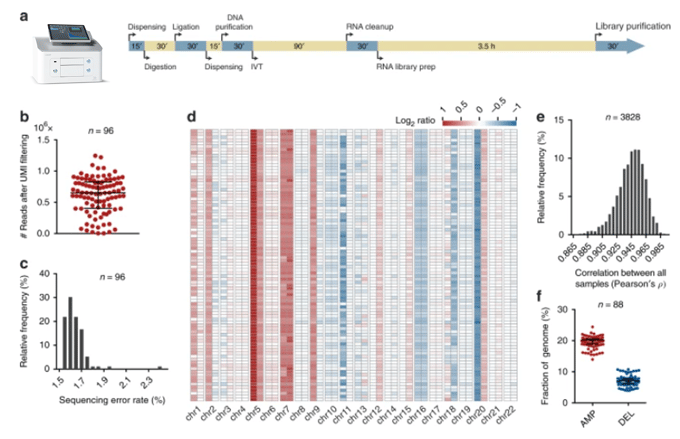
Figure 2. High-throughput CUTseq. a The I.DOT Non-Contact Dispenser, a low-volume non-contact liquid handling device (DISPENDIX) that was used in this and previous studies by this research team, and timeline for high-throughput CUTseq library preparation. IVT, in vitro transcription. The total workflow takes ~8 h for a single person to prepare 1–2 libraries, each containing up to 96 samples. The dispensing step can be done either manually or using a liquid handling device such as the I.DOT. b Number of usable reads (after alignment and PCR duplicates removal) per sample, in one multiplexed CUTseq library prepared from 96 replicate samples (n) of HeLa cells gDNA (5 ng), using the I.DOT. c Distribution of the sequencing error rates in the 96 replicates (n) shown in b. d Copy number profiles (1 Mb resolution, averaged at arm level for visualization) of 88 replicates shown in b that yielded at least 300 K usable reads. The remaining eight samples were not included, as the number of usable reads was insufficient to perform reliable copy number calling. e Distribution of all possible (n) pairwise Pearson’s correlations between the copy number profiles shown in d. f Fractions of the genome either amplified (AMP) or deleted (AMP) in the 88 replicates (n) shown in d. Each dot represents one sample. Error bars indicate the median and interquartile range. Figure modified from Zhang, X., Garnerone, S., Simonetti, M. et al. (2019).
Conclusion
The integration of the I.DOT Liquid Handler with high-throughput CUTseq is a game-changer in genomic research. By improving efficiency, accuracy, and scalability, this combination empowers researchers to delve deeper into the complexities of the genome, paving the way for groundbreaking discoveries in biology and medicine.
References
Zhang, N., Harbers, L., Simonetti, M. et al. High clonal diversity and spatial genetic admixture in early prostate cancer and surrounding normal tissue. Nat Commun 15, 3475 (2024). https://doi.org/10.1038/s41467-024-47664-z
Zhang, X., Garnerone, S., Simonetti, M. et al. CUTseq is a versatile method for preparing multiplexed DNA sequencing libraries from low-input samples. Nat Commun 10, 4732 (2019). https://doi.org/10.1038/s41467-019-12570-2