Miniaturized assays represent a revolutionary advancement in scientific research, substantially reducing the scale of experimental setups while maintaining accuracy and reproducibility. Not only does miniaturization conserve valuable resources such as reagents and samples, but it also enables increased assay throughput, improving productivity and accelerating innovation and discovery across the biomedical sciences.
Applications of Miniaturized Assays
In recent years, advanced tools and methodologies have broadened the application of miniaturized assays across various fields, showing notable promise in clinical research, including drug discovery, diagnostics, and personalized medicine1.
Drug Discovery
Drug discovery is an inherently complex, costly, and time-consuming process that employs high-throughput screening (HTS) at several stages throughout the process. Miniaturized assays allow researchers to reduce the sample volume significantly and increase the scale of cell-based microplate assays, including cytotoxicity, enzyme activity, and binding assays, from 96 wells to 384, 1536, or even 3456 wells2. Conducting these assays in a high-throughput format is important for allowing researchers to test thousands of candidate compounds rapidly. Additionally, the advent of microphysiological systems – organ-on-chip (OOC) and, more recently, lab-on-a-chip (LOC) – have proven helpful for disease modeling and toxicity testing, reducing reliance on animal testing and providing researchers with cost-efficient, effective, and ethical alternatives3,4 (Fig. 1).
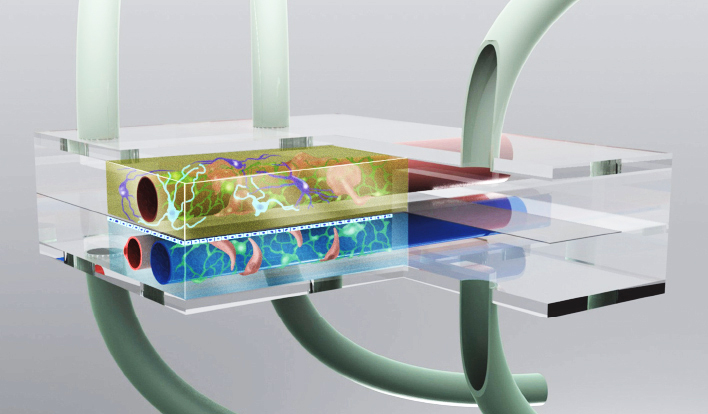
Figure 1. Microphysiological systems such as organ-on-chip and lab-on-a-chip systems allow small-scale disease modeling. (Source)
Diagnostics
In diagnostics, miniaturized assays facilitate the development of compact, rapid, and sensitive tests crucial for early disease detection. Diagnostics-based miniaturization approaches include microfluidics and miniaturized fluidics-based platforms, which have allowed the precise testing of tiny volumes of body fluids without the need for large, expensive, specialized equipment, enabling rapid, accurate point-of-care diagnoses5 (Fig. 2). Moreover, miniaturization of genomics assays such as next-generation sequencing (NGS) allows robust, cost-efficient DNA, RNA, and targeted sequencing for clinical diagnostics. With the correct tools and technologies, researchers can scale NGS samples as low as one-tenth of the recommended volumes without compromising data quality.
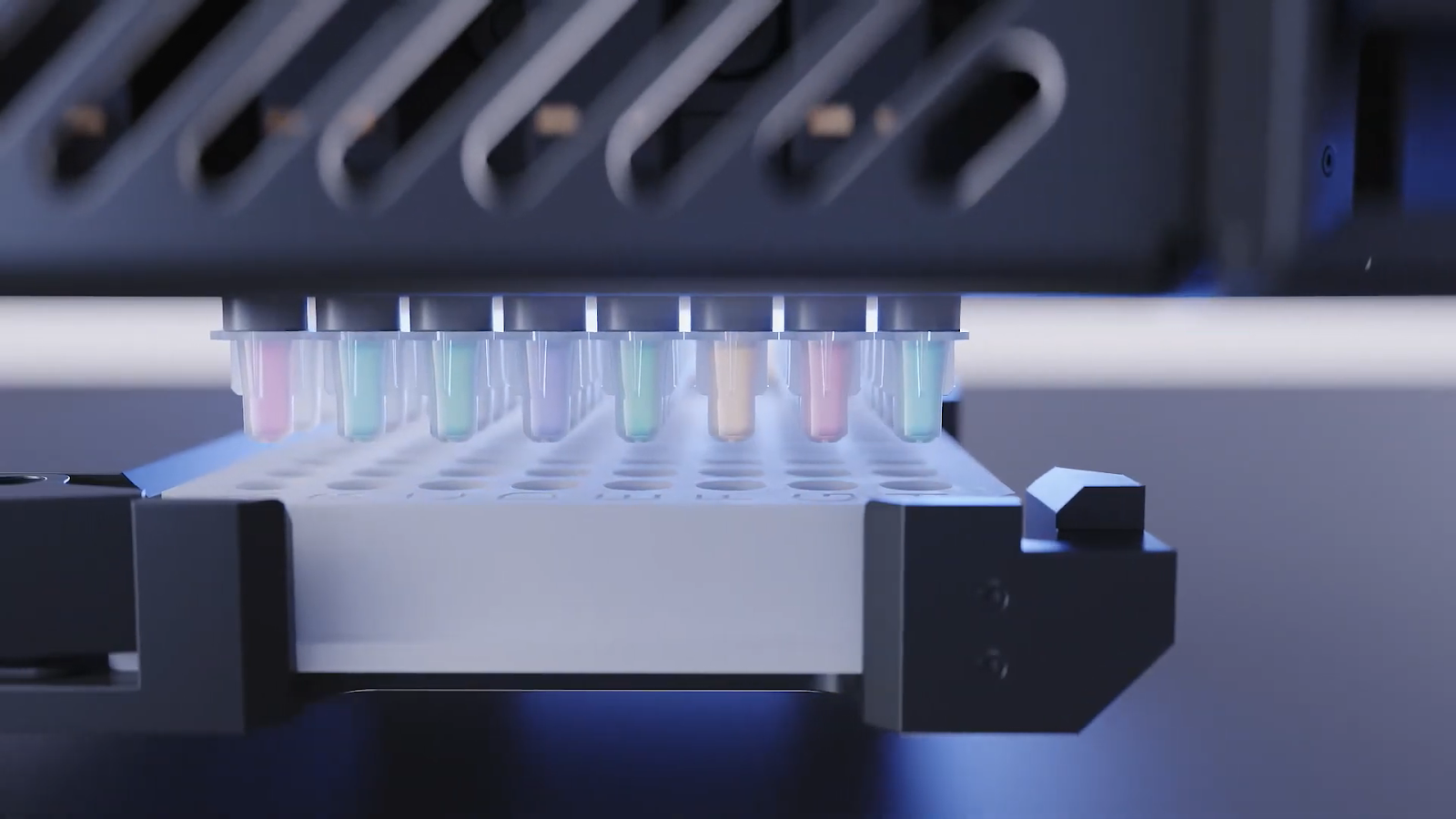
Figure 2. Automated liquid handling, like the I.DOT Liquid Handler, streamlines high-throughput sequencing workflows.
Personalized Medicine
In personalized medicine, miniaturized assays offer a strategy to tailor treatments to individual patients, marking a significant step towards more effective and bespoke healthcare solutions6,7. The key benefit to using miniaturized assays in personalized medicine is the capacity to perform a range of essential assays with a tiny amount of starting material. This is a game-changer for healthcare workers, who can now accurately diagnose conditions and spot those at risk of hereditary disorders. What’s more, miniaturized assays, such as genomic sequencing, tile-based spatial transcriptomics, microfluidics approaches, and cell-based microplate assays, help clinicians monitor the patient’s disease progression and response to treatments, empowering medics to make well-informed clinical decisions.

Challenges and Future Directions
While assay miniaturization holds great promise, it faces several challenges1,8:
- Protocol Optimization: Successful miniaturization requires protocols to be carefully adapted for smaller formats. This often involves addressing complexities in liquid handling, which can be more challenging with smaller sample volumes – utilizing automated liquid handling systems such as the I.DOT Liquid Handler and selecting the correct hardware are essential steps. While many large pharmaceutical companies are shrinking the scale of their chemical reactions through miniaturization, this process can be tricky when it comes to maintaining high-quality data. The DISPENDIX team has extensive experience miniaturizing even the most complex DNA sequencing workflows. We can help you reduce your reaction volumes by 90% compared to standard protocols, all while ensuring your data stays accurate and reliable.
- Assay Validation: Post-miniaturization, it's crucial to validate assays to ensure they maintain the accuracy and robustness of traditional methods. This step is time-consuming but vital for reliable results.
- Assay Standardization: Scaling up assays increases the risk of human error, demanding standardization, especially in critical areas like drug discovery, diagnostics, and personalized medicine. Automation in liquid handling, sample cleanup, and library preparation helps achieve consistent results and minimizes variability.
- Data Management: With larger-scale assays comes increased volumes of data, which can be challenging to manage and analyze, requiring highly skilled analysts and bioinformaticians. Advanced AI-based analysis tools are, however, simplifying this process and reducing the workload involved in data interpretation, streamlining the process of gaining meaningful insights from HTS data.
In the world of biomedical sciences, miniaturized assays are gaining in popularity, especially among clinical researchers, healthcare professionals, and drug discovery scientists. We still have a long way to go before miniaturization reaches its full potential, with ongoing research and development efforts to refine protocols and develop new technologies to improve the process. However, it is not just about advancements in the lab; progress in this field calls for ongoing collaboration, innovation, and a drive to shape the future of healthcare.
Conclusion
Overall, miniaturized assays have proven to be a game-changer in scientific research. They’ve brought a whole new level of efficiency while bringing down costs, fast-tracking drug discovery and diagnostics, and improving personalization in medicine. Looking ahead, we predict further exciting advancements in this field that will likely transform the biomedical sciences and pave the way for more targeted, tailored healthcare.

Ready to elevate your workflow with reliable and reproducible miniaturized assays? Book a consultation with DISPENDIX today and explore how the I.DOT Liquid Handler can accelerate your research, improve lab sustainability, and generate results that you can trust. Experience precision and efficiency that can transform your research, from drug discovery to diagnostics and beyond. Get in touch now to learn more from our automation experts!
References
-
Pereira SAP, Dyson PJ, Saraiva MLMFS. Miniaturized technologies for high-throughput drug screening enzymatic assays and diagnostics – A review. TrAC Trends Anal Chem. 2020;126:115862. doi:10.1016/j.trac.2020.115862
-
Carstens C, Elbracht R, Gärtner C, Becker H. Opportunities and limits of cell-based assay miniaturization in drug discovery. Expert Opin Drug Discov. 2010;5(7):673-679. doi:10.1517/17460441.2010.488264
-
Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA. Organs-on-chips: into the next decade. Nat Rev Drug Discov. 2021;20(5):345-361. doi:10.1038/s41573-020-0079-3
-
Sung JH, Wang YI, Narasimhan Sriram N, et al. Recent Advances in Body-on-a-Chip Systems. Anal Chem. 2019;91(1):330-351. doi:10.1021/acs.analchem.8b05293
-
Kricka LJ, Park JY, Li SF, Fortina P. Miniaturized detection technology in molecular diagnostics. Expert Rev Mol Diagn. 2005;5(4):549-559. doi:10.1586/14737159.5.4.549
-
Natalia A, Zhang L, Sundah NR, Zhang Y, Shao H. Analytical device miniaturization for the detection of circulating biomarkers. Nat Rev Bioeng. 2023;1(7):481-498. doi:10.1038/s44222-023-00050-8
-
Grandori C, Kemp CJ. Personalized Cancer Models for Target Discovery and Precision Medicine. Trends Cancer. 2018;4(9):634-642. doi:10.1016/j.trecan.2018.07.005
-
Nickischer D, Elkin L, Cloutier N, O’Connell J, Banks M, Weston A. Challenges and Opportunities in Enabling High-Throughput, Miniaturized High Content Screening. In: Johnston PA, Trask OJ, eds. High Content Screening. Vol 1683. Methods in Molecular Biology. Springer New York; 2018:165-191. doi:10.1007/978-1-4939-7357-6_11