In the rapidly evolving field of microbial ecology, advancements in DNA sequencing have dramatically expanded our understanding of microbial communities. However, the cost and efficiency of sample preparation and library creation remain significant barriers, particularly for large-scale projects.
A recent study High-throughput DNA extraction and cost-effective miniaturized metagenome and amplicon library preparation of soil samples for DNA sequencing from our customers at Aalborg University Center for Microbial Communities, led by Thomas BN Jensen and colleagues, introduces a game-changing approach that leverages the innovative capabilities of the I.DOT Liquid Handler, a nanoliter dispensing robot. This non-contact technology is poised to revolutionize the way researchers conduct high-throughput DNA extraction and library preparation, making these processes more cost-effective and efficient.

Benchmarking High-Throughput DNA Extraction Methods
The study conducted by the Aalborg University team evaluated three high-throughput DNA extraction kits: the ZymoBIOMICS™ 96 MagBead DNA Kit, MP Biomedicals™ FastDNA™-96 Soil Microbe DNA Kit, and the DNeasy® 96 PowerSoil® Pro QIAcube® HT Kit. These kits were tested on five diverse soil types to assess their performance in terms of DNA yield, quality, and the resulting microbial community profiles (Fig. 1).
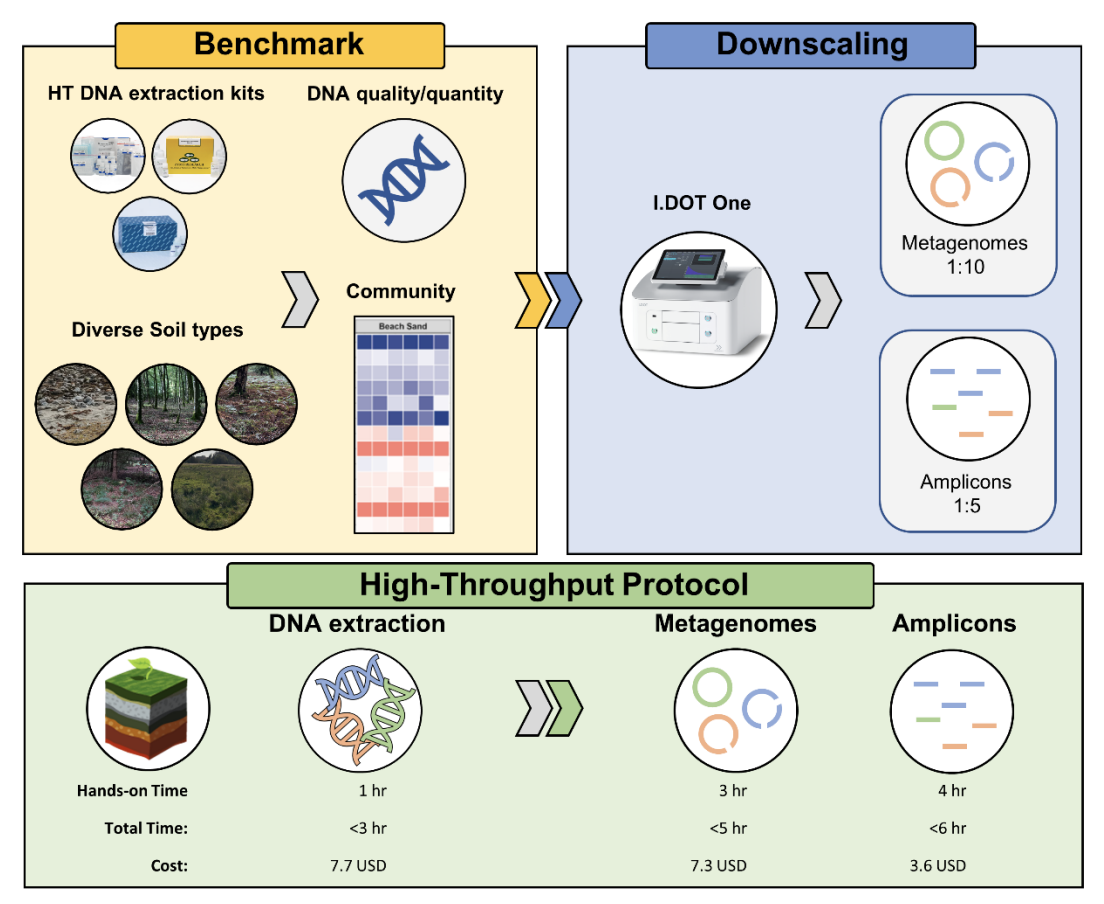
Figure 1. This experiment compared three high-throughput DNA extraction methods for analyzing microbial communities in five soil types. The researchers assessed extracted DNA quality, quantity, and ability to represent the community. The I.DOT Liquid Handler then miniaturized library preparation for metagenomes and amplicons. The authors further analyzed hands-on time, total processing time, and cost per sample for each step, from DNA extraction to final product. Notably, two 96-well plates can be processed concurrently for both metagenomes and amplicons. This approach offers faster analysis, reduced materials usage, and cost savings compared to traditional methods.
Among the kits tested, the DNeasy® 96 PowerSoil® Pro QIAcube® HT Kit demonstrated superior performance across all soil types, producing high-quality DNA with minimal contamination. This kit's ability to efficiently process diverse soil samples makes it a valuable tool for microbial ecology research.
Miniaturization with the I.DOT Liquid Handler
The real breakthrough in the study comes with the introduction of the I.DOT Liquid Handler, which the researchers used to miniaturize the volumes required for Illumina amplicon and metagenomic library preparation.
By reducing the reaction volumes by factors of 5 and 10 for amplicon and metagenome libraries, respectively, the I.DOT Non-Contact Dispenser significantly cut down the costs of chemicals and plastics, from $5.00 to $3.60 and $59.00 to $7.30 per sample (Fig. 1).
This miniaturization did not compromise the quality of the libraries or the accuracy of the microbial community profiles. The I.DOT Non-Contact Dispenser enabled the preparation of a full 96-well plate in approximately three hours for DNA extraction, five hours for metagenome library preparation, and six hours for amplicon library preparation.
These time savings, combined with reduced costs, make high-throughput sequencing more accessible and feasible for large-scale projects.
Impact on Microbial Ecology Research
The implications of this study are profound for the field of microbial ecology. The ability to efficiently and cost-effectively prepare large numbers of samples for sequencing opens up new possibilities for researchers. Projects that were previously limited by budget and labor constraints can now be undertaken, leading to a deeper understanding of microbial diversity and its ecological roles.
The I.DOT's role in this transformation cannot be overstated. Its precision and efficiency in dispensing nanoliter volumes make it an invaluable tool for researchers looking to optimize their workflows and reduce costs without sacrificing quality.
Conclusion
The research conducted by Jensen and colleagues highlights the transformative potential of integrating high-throughput DNA extraction methods with innovative technologies like the I.DOT Non-Contact Dispenser.
By miniaturizing the library preparation process, the I.DOT Non-Contact Dispenser not only reduces costs but also enhances the efficiency and scalability of microbial ecology studies. As sequencing technologies continue to advance, the adoption of tools like the I.DOT will be crucial in driving forward our understanding of the microbial world.
For more details on this groundbreaking research, you can read/download the full paper here.
Experience unparalleled efficiency and cost savings in high-throughput DNA extraction and library preparation. Elevate your research capabilities — book a demo of the I.DOT Liquid Handler today!
References
Jensen, T. B. N., Kirkegaard, R. H., Dueholm, M. S., Brink, L. H., Munck, C., Kusk, H. H., & Nielsen, P. H. (2023). High-throughput DNA extraction and library preparation of soil samples reveal the benefits of miniaturization using I.DOT. bioRxiv. https://doi.org/10.1101/2023.09.04.556179