Traditional, centralized pharmaceutical production struggles to meet the growing demand for localized, personalized medicine. Distributed and Point-of-Care (POC) manufacturing facilities offer a more agile solution, catering to specific needs like narrow therapeutic index drugs and specialized pediatric or geriatric dosing.
This shift is driven by advancements in technologies like 3D printing and Immediate Drop-On-Demand Technology (I.DOT) dispensing. Researchers, our customers, at the National Institute of Standards and Technology propose a framework based on the Quality by Design (QbD) principles to ensure consistent quality within this new paradigm (Fig. 1).

Figure 1. The working paper Quality by Design Considerations for Drop-on-Demand Point-of-Care Pharmaceutical Manufacturing of Precision Medicine by researchers at the National Institute of Standards and Technology.
How It Works
- Centralized API Production: Active pharmaceutical ingredients (APIs) are manufactured in a controlled, centralized facility under strict quality control (QC) measures and with the use of the I.DOT Liquid Handler (Fig. 2). These APIs are essentially transformed into "inks" for use in point-of-care (POC) settings.
- Distributed "Ink" and POC Dispensing: The API inks are then distributed to POC locations like hospitals or pharmacies. Here, the I.DOT Liquid Handler precisely dispenses the required dosage form onto or into delivery vehicles such as single-dose vials, capsules, or orodispersible films (Fig. 2).
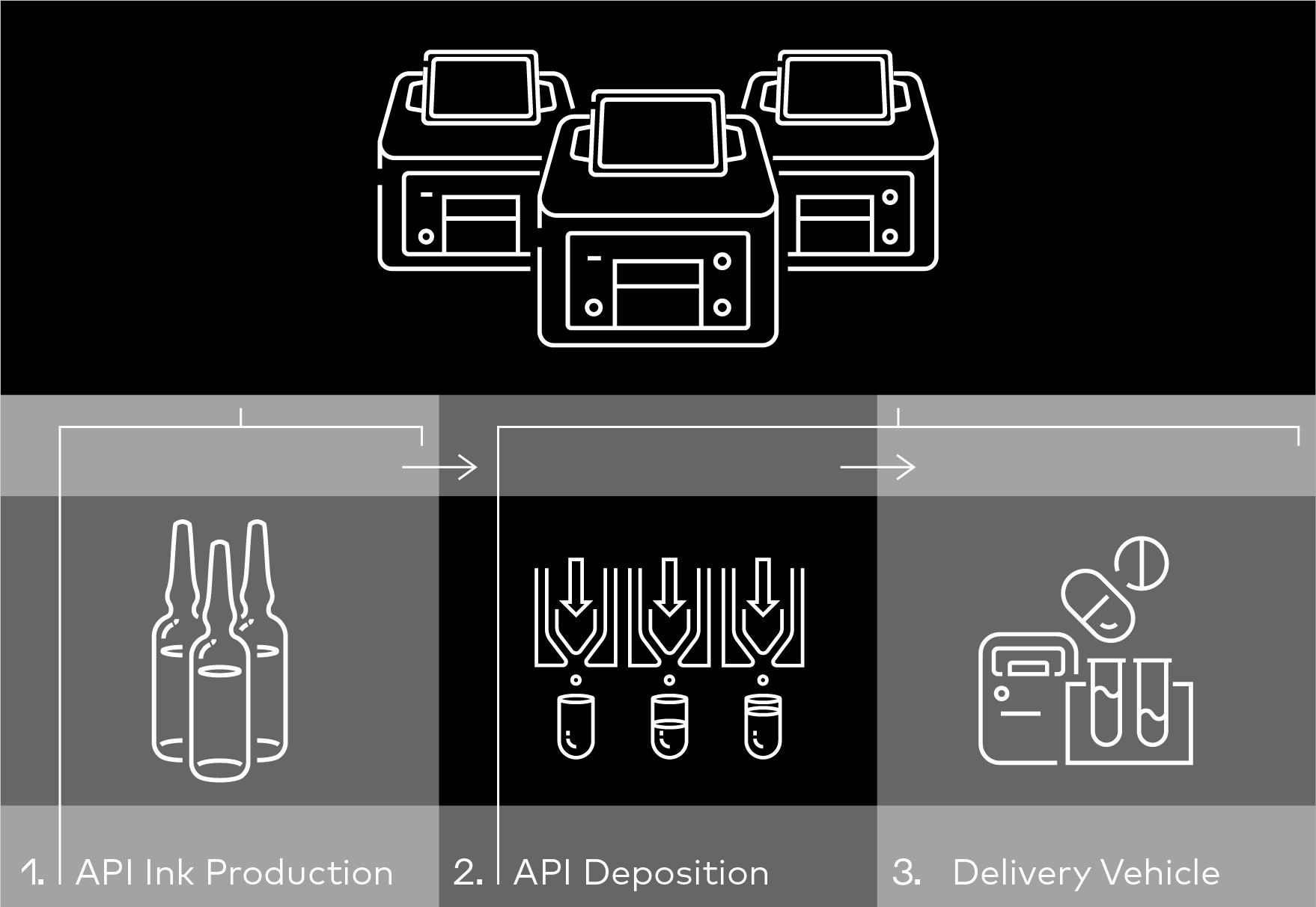
Figure 2. API (active pharmaceutical ingredient) ink production at a centralized manufacturing facility, API deposition onsite by drop-on-demand dispensing using the I.DOT Non-Contact Dispenser, and delivery vehicle and mechanism consideration (e.g., single-dose vials, capsules, or orodispersible film, etc.) steps of the point-of-care (POC) manufacturing processes.
The I.DOT Liquid Handler is a non-contact, drop-on-demand liquid handler that delivers precise amounts of APIs (Fig. 2). It works by using tiny bursts of room-temperature air pressure (lasting microseconds) to eject individual droplets from a special 96-well plate down onto a target plate.
Depending on settings like pressure and pulse duration, the I.DOT Non-Contact Dispenser can dispense individual droplets ranging from 10 nanoliters (nL) to 50 nL each. The source plate comes in two versions with different nozzle sizes (100 µm and 60 µm diameter) – the S.100 and S.60 dispense plates, respectively. Each well in the plate can hold up to 80 µL of liquid and is linked to one of eight independent pressure channels, allowing for individual control of each well.
The I.DOT Non-Contact Dispenser is designed to work with various types of liquids and can be calibrated for accurate dispensing. It even has a built-in feature called DropDetection that alerts users when a source well is running low on liquid.

Quality Control at POC Sites
Since full-fledged QC labs are impractical at each POC site, the researchers propose a multi-pronged control strategy:
- Atline Verification: Before dispensing, the API ink undergoes UV-Vis spectroscopy to confirm its properties.
- Inline Monitoring: During dispensing, the system automatically counts each drop for accuracy.
- Intermediate Checks: Dispensed volume is verified at specific points during the process.
- Offline Confirmation: After production, a representative sample from each batch undergoes LC-MS/MS analysis for final confirmation.
This pilot project using levothyroxine sodium for single-dose liquid vials (prepared with glycerin/water) successfully achieved consistent quality within established acceptance values. This study demonstrates the potential of the proposed framework for reliable POC manufacturing of personalized medications.
Ready to revolutionize your approach to pharmaceutical manufacturing?
The I.DOT Liquid Handler is at the forefront of the shift towards agile, POC production.
This innovative technology offers:
- Precise, on-demand API dispensing: Deliver personalized medicine with unmatched accuracy.
- Enhanced flexibility: Adapt to individual patient needs and streamline workflows.
- Reduced waste: Minimize material usage for a more sustainable approach.
See I.DOT in action! Schedule your free, personalized demo today and discover how it can transform your lab for reliable POC production.
References
Forbes TP, Gillen JG, Feeney W, Ho J. Quality by Design Considerations for Drop-on-Demand Point-of-Care Pharmaceutical Manufacturing of Precision Medicine. ChemRxiv. 2024; doi:10.26434/chemrxiv-2024-20znb This content is a preprint and has not been peer-reviewed.