Protocol Exchange recently published scCUTseq is a versatile framework for spatially resolved single-cell genomic analyses of intratumor heterogeneity which addresses the single-cell CUT sequencing (scCUTseq) by utilizing Immediate Drop on Demand Technology (I.DOT) for miniaturizing reaction volumes (Fig. 1).
Figure 1. The paper scCUTseq is a versatile framework for spatially resolved single-cell genomic analyses of intratumor heterogeneity in Protocol Exchange.
The researchers from Karolinska Institute and University of Turin, Italy leverage the automated, high-throughput, intuitive, and sustainable technology for single-cell genomic analyses of intratumor heterogeneity (ITH). The authors are impressed by the I.DOT's processes of automation, prospective of versatility, accuracy, reproducibility, and increased flexibility in dispensing low volumes throughout the scCUTseq workflow.
Comprising billions of cells, tumors are intricate ecosystems that undergo continuous evolution over time. Although the precise mechanism underlying the development of these tumoral masses is still unknown, numerous efforts have been made over the years to identify the critical moments that turn a normal cell malignant. It is now widely accepted that tumor development is a multi-step process in which genetic alterations result in mutations that provide cells with benefits.
Moreover, the notion of cancer hallmarks was introduced, marking a significant turning point in the field of tumor biology. (Hanahan and Weinberg., 2000). The diversity of this complex environment can be quantified at both the genetic and phenotypic levels and are classified as inter-tumor heterogeneity and intra-tumor heterogeneity or ITH. While the latter denotes differences within the same tumor, the former speaks of differences between tumors of the same type but of different individuals.
In addition to methods for examining morphological ITH, microscopy or sequencing techniques can be used to study genetic heterogeneity. The inability to generate substantial amounts of information in a single experiment and their low throughput are the main limitations of microscopic tools. On the other hand, sequencing techniques enable the simultaneous analysis and measurement of a large number of genes and transcripts. Furthermore, thanks to a new generation of sequencing tools, that allows studying tumor composition, evolution, and alterations at unprecedented scale.
Exciting Advancements in Single-Cell Genomics
Low-input genomic deoxyribonucleic acid (gDNA) samples can be amplified and tagged using the reduced representation genome sequencing technique CUTseq to create multiplexed NGS libraries. Copy number alterations (CNAs) in gDNA extracted from cell lines and formalin-fixed paraffin embedded (FFPE) tissue specimens can be reliably studied using this method.
Recently, scientists profiled genetic ITH in multiple regions of FFPE sections of primary and metastatic breast cancer lesions using the CUTseq method (Zhang et al., 2019). Furthermore, an advanced technique that expands upon the architecture of CUTseq, that includes a whole-genome amplification (WGA) step to amplify gDNA from single-cells was also developed (Zhang et al., 2023). The authors used the I.DOT Liquid Handler to dispense various reagents throughout the entire process of generating high-quality libraries for the scCUTseq, and its high-throughput version. This reduced the volume of reactions to nanoliters (1:200), lowered the reagent costs, and increased the number of cells that could be analyzed in parallel.
Finally, to test the method's sensitivity after miniaturizing reaction volumes with I.DOT, the authors were able to verify a 7 megabase (Mb) deletion that occurs at an extremely low rate (4.5%) among human TK6 lymphoblastoid cells.
Single-cell studies unravel the intricacies of cellular heterogeneity. In the realm of epigenetics, the fusion of the I.DOT with scCUTseq takes this exploration to new heights.
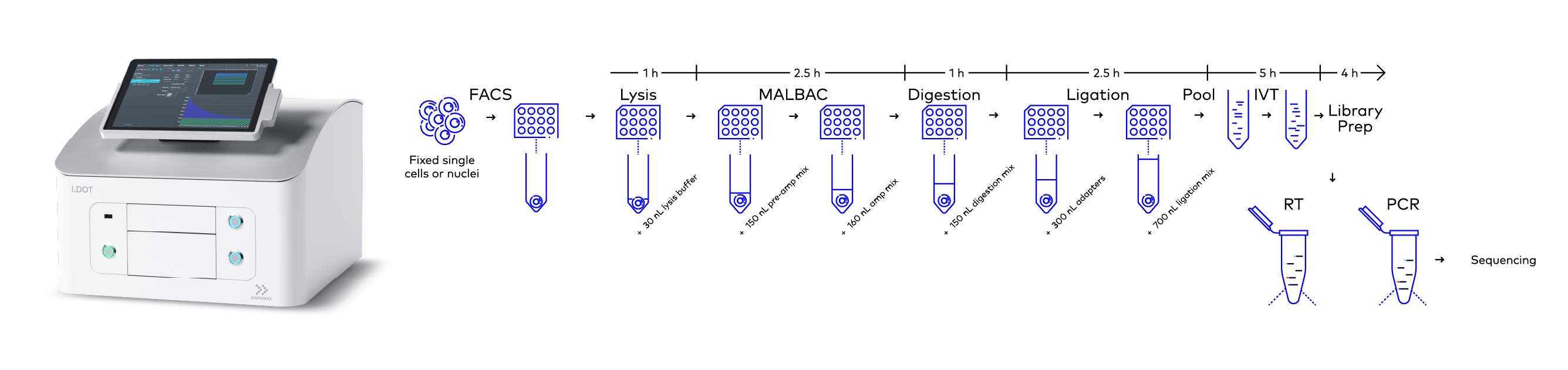
Figure 2. Workflow of scCUTseq. Briefly, fixed single cells or nuclei are FACS sorted into a multi-well plate and WGA is carried out using MALBAC in three steps: lysis, pre-amplification, and amplification. Digestion, ligation, and library preparation are then performed. The dispensing of all the reagents in these processes was done using the I.DOT Liquid Handler. Image modified from Michele Simonetti, Ph.D. thesis, 2021.
The library preparation workflow could not have been miniaturized by 1:200 as efficiently or accurately without the inclusion of the I.DOT Non-Contact Dispenser, which was used to precisely dispense various reagents throughout the scCUTseq processes (Fig. 2). The liquid handling steps for the scCUTseq include:
- Cell lysis: 30 nL of lysis mix was dispensed using the I.DOT liquid handler
- Pre-amplification: Dispensing the pre-amplification mix 150 nL
- Amplification: Dispensing 150 nL of amplification mix including 11 nL of 4x SYBR Green (Thermo Fisher Scientific, Cat. no. S7563)
- Digestion: Dispensing 150 nL of restriction digestion enzyme mix (NlaIII, NEB, Cat. no. R0125L)
- Ligation: 700 nL of ligation mix including 300 nL of scCUTseq adapters were dispensed
- In vitro transcription (IVT): Dispensing MEGAscript® T7 Transcription kit (Thermo Fisher Scientific, Cat. no. AM1334-5) reagents in microliter range accurately
- Library preparation: Dispensing TruSeq Small RNA Library Preparation kit reagents (Illumina, Cat. no. RS-200-0012/RS-200-0024) in nanoliter to microliter range
As Technology Evolves, The Horizon Expands
Anticipate further breakthroughs using non-contact low volume I.DOT Liquid Handler, driving innovation in personalized medicine and disease therapeutics. Besides the extreme importance of I.DOT Liquid Handler in scCUTseq in cancer biology, I.DOT’S versatility can also play a pivotal role in studying viral genomes, like SARS-CoV-2, for tracking mutations and understanding host-virus interactions. Moreover, monitoring novel variants is crucial, potentially impacting the virus's susceptibility to recently developed vaccinations.

Embark on a journey into the next frontier of single-cell research with our groundbreaking product that highlights the power of the I.DOT in scCUT sequencing. Their synergy accelerates the understanding of cellular dynamics, contributing significantly to disease research, viral evolution studies, and beyond!
Book a demo today!
References
Hanahan, D. and Weinberg, R.A., 2000. The hallmarks of cancer. Cell, 100(1), pp.57-70.
Zhang, X., Garnerone, S., Simonetti, M., Harbers, L., Nicoś, M., Mirzazadeh, R., Venesio, T., Sapino, A., Hartman, J., Marchiò, C. and Bienko, M., 2019. CUTseq is a versatile method for preparing multiplexed DNA sequencing libraries from low-input samples. Nature Communications, 10(1), p.4732.
Zhang, Ning & Simonetti, Michele & Harbers, Luuk & Helleday, Thomas & Roukos, Vassilis & Marchiò, Caterina & Bienko, Magda & Crosetto, Nicola. 2021. scCUTseq is a versatile framework for spatially resolved single-cell genomic analyses of intratumor heterogeneity. 10.21203/rs.3.pex-1602/v1.