Next-generation sequencing (NGS) is a method of sequencing DNA whereby millions of fragments of DNA are sequenced in parallel and pieced together using bioinformatics to form an entire genome or specific area of interest1. NGS has been growing in popularity since its inception, overtaking Sanger sequencing in popularity, due to its cost-effectiveness, sensitivity, and increased throughput2. NGS now has widespread uses across biology and healthcare, including in identifying genetic disease determinants and characterizing cancers for personalized treatment3.
NGS automation is gaining popularity as a method of managing complex, multi-stepped workflows and increasing sample throughput. Accuracy and reproducibility are key to NGS, making automated sample preparation a crucial component in increasing the efficiency of NGS4.
Challenges in Manual NGS Sample Prep and Workflow Bottlenecks
Multiple stages in NGS are labor-intensive, including sample preparation, library preparation and PCR. When carrying these processes out manually, researchers have to be hands-on for long periods of time, limiting their ability to carry out any other tasks5. Attempting to speed up manual sample prep can result in an increased likelihood of human error and contamination. In subsequent stages of NGS, the DNA is amplified resulting in error amplification and PCR-exacerbated bias6,7.
In addition, manual sample prep produces challenges with reproducibility. Scaling experiments up for large studies or clinical applications requires consistency, which is a challenge when carrying out manual sample prep as techniques vary from person to person. For example, variations in manual pipetting techniques are common8 and can result in a batch effect and incorrect conclusions being drawn9.
Benefits of Integrating Automated Sample Prep in NGS Automation
NGS automation is increasing in popularity as a method of addressing issues with manual sample preparation. One example is DISPENDIX’s NGS Library Preparation systems, including the I.DOT Liquid Handler (Fig. 1) and the G.PURE NGS Clean-Up Device, both of which are included in the G.PREP NGS Bundle.
Figure 1. The I.DOT Liquid Handler can be used in library preparation to quickly and precisely dispense small volumes of reagents, including enzymes, beads, or buffers, to samples.


Enhanced Precision and Reproducibility
The use of NGS automation reduces the need for manual sample processing by up to 80%. NGS automation therefore enhances the accuracy and precision of the results by reducing the probability of human error5,10. The consistency achieved through the use of NGS automation improves experiment reproducibility as the removal of researcher-to-researcher differences reduces the batch effect.
Increased Throughput
The use of manual techniques can decrease NGS throughput as humans are limited in how many samples we can successfully process at once and how fast we can work. NGS automation increases throughput as it enables many samples to be processed simultaneously instead of sequentially11. The time taken for sample preparation is therefore decreased and furthermore, the number of samples processed can be increased.
Reduced Contamination Risks
Human errors in the manual processing of samples can result in sample contamination and subsequently to erroneous data7,12. NGS automation reduces the risk of human handling errors. In addition, due to the closed nature of the automated process, human intervention and environmental exposure are minimized10. Many NGS automation systems are made to be non-contact - this ability to dispense liquid without the need for pipette tips and without touching the well further reduces contamination (Fig. 2).
Figure 2. The I.DOT Liquid Handler dispenses liquid at a rate of 100 droplets per second per channel using non-contact liquid handling. 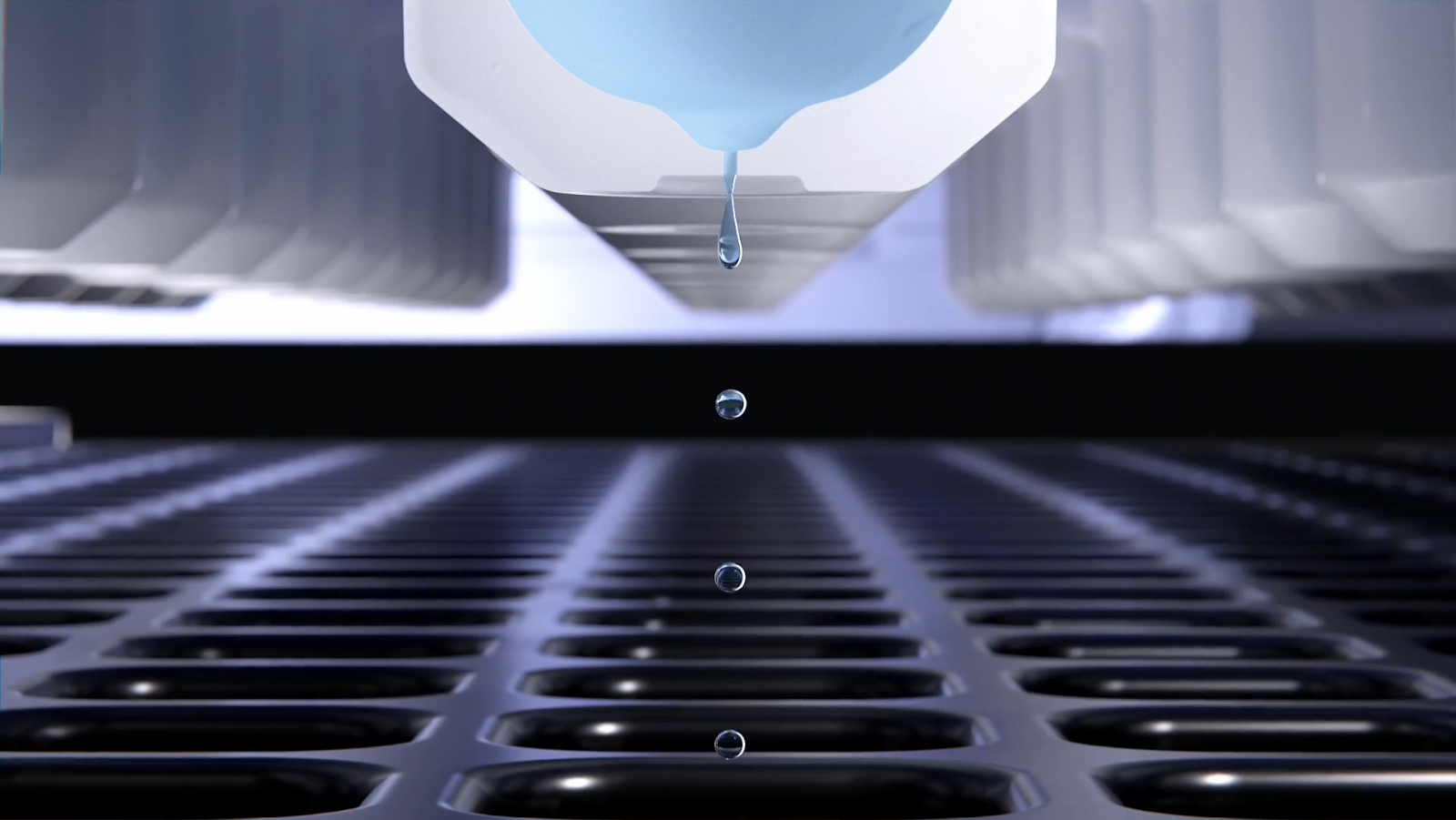
How to Integrate Automated Sample Prep into NGS Workflows
When identifying a suitable system for NGS automation, researchers should consider their key lab needs. These should include:
- Throughput requirements - how many samples need to be prepared and analyzed simultaneously
- Precision/accuracy requirements - if the data is to be used to drive clinical decisions then the accuracy needs to be very high13
- Sample volume requirements - clinical samples may come in very small volumes so the NGS automation system must be capable of working with the minuscule volumes provided14.
Researchers should also consider which part of the NGS workflow they want to automate - different NGS automation equipment will be suitable for different stages of the workflow. For example, DISPENDIX produces the I.DOT Liquid Dispenser to dispense enzymes, beads, and buffers into samples, and the G.PURE which performs miniaturized bead clean-ups. These NGS automation systems not only speed up and improve the accuracy of NGS but can also decrease the costs associated with NGS (see how much your lab could save using the G.PREP ROI calculator).
Researchers should ensure that any automation systems used are compatible with any pre-existing software. In addition, data management should be considered - this could include whether labs require barcode scanning/tracking and integration with laboratory information management systems (LIMS), this can be particularly helpful in NGS automation systems if using clinical samples.
Conclusion
NGS requires extensive and time-consuming sample preparation. NGS automation is increasingly being used to meet the challenges associated with manual sample processing. The automation of sample preparation can increase throughput by simultaneously processing multiple samples, increase precision and reproducibility by reducing human error and providing consistency between experiments, and decrease contamination by providing a closed system with non-contact dispensing solutions. NGS automation therefore increases efficiency and accuracy, and allows experiments to be scaled up for large-scale projects.
Ready to streamline your NGS workflows?
Discover how G.PREP NGS Automation can transform your lab’s efficiency and precision. With advanced automated sample prep, G.PREP ensures consistency, minimizes contamination, and maximizes throughput. Start optimizing your sequencing process today — download the G.PREP brochure and see the difference automation can make!
References
- Behjati S, Tarpey PS. What is next generation sequencing? Arch Dis Child Educ Pract Ed. 2013;98(6):236. doi:10.1136/archdischild-2013-304340
- Ari Ş, Arikan M. Next-Generation Sequencing: Advantages, Disadvantages, and Future. In: Hakeem KR, Tombuloğlu H, Tombuloğlu G, eds. Plant Omics: Trends and Applications. Springer International Publishing; 2016:109-135. doi:10.1007/978-3-319-31703-8_5
- Zhong Y, Xu F, Wu J, Schubert J, Li MM. Application of Next Generation Sequencing in Laboratory Medicine. Ann Lab Med. 2021;41(1):25-43. doi:10.3343/alm.2021.41.1.25
- Cheng C, Fei Z, Xiao P. Methods to improve the accuracy of next-generation sequencing. Front Bioeng Biotechnol. 2023;11:982111. doi:10.3389/fbioe.2023.982111
- Socea JN, Stone VN, Qian X, Gibbs PL, Levinson KJ. Implementing laboratory automation for next-generation sequencing: benefits and challenges for library preparation. Front Public Health. 2023;11:1195581. doi:10.3389/fpubh.2023.1195581
- Head SR, Komori HK, LaMere SA, et al. Library construction for next-generation sequencing: Overviews and challenges. BioTechniques. 2014;56(2):61. doi:10.2144/000114133
- Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12(1):87. doi:10.1186/s12915-014-0087-z
- Guan XL, Chang DPS, Mok ZX, Lee B. Assessing variations in manual pipetting: An under-investigated requirement of good laboratory practice. J Mass Spectrom Adv Clin Lab. 2023;30:25-29. doi:10.1016/j.jmsacl.2023.09.001
- Leek JT, Scharpf RB, Bravo HC, et al. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet. 2010;11(10):733-739. doi:10.1038/nrg2825
- Hess JF, Kohl TA, Kotrová M, et al. Library preparation for next generation sequencing: A review of automation strategies. Biotechnol Adv. 2020;41:107537. doi:10.1016/j.biotechadv.2020.107537
- O’Neill MD. Increasing Throughput with Automated Liquid Handling. Genet Eng Biotechnol News. 2012;32(12):18-21. doi:10.1089/gen.32.12.06
- Kotrova M, Trka J, Kneba M, Brüggemann M. Is Next-Generation Sequencing the way to go for Residual Disease Monitoring in Acute Lymphoblastic Leukemia? Mol Diagn Ther. 2017;21(5):481-492. doi:10.1007/s40291-017-0277-9
- Karimnezhad A, Palidwor GA, Thavorn K, et al. Accuracy and reproducibility of somatic point mutation calling in clinical-type targeted sequencing data. BMC Med Genomics. 2020;13(1):156. doi:10.1186/s12920-020-00803-z
- Parekh M, Borroni D, Romano V, et al. Next-generation sequencing for the detection of microorganisms present in human donor corneal preservation medium. BMJ Open Ophthalmol. 2019;4(1):e000246. doi:10.1136/bmjophth-2018-000246