Advances in scientific research have resulted in an increased number of high-throughput and sample-heavy experiments with complex workflows1. When carried out manually, these experiments are labor-intensive, time-intensive, prone to human error, and lack the consistency required to scale up experiments for large studies or clinical applications2.
As a result, automated sample preparation systems are becoming more popular as a way of driving productivity, accuracy, and consistency. An increasing number of labs are looking to invest in these systems as a way to expand their high-throughput capabilities. One such system is DISPENDIX’s G.PREP NGS Automation which uses standardized methods and precise reagent dispensing to optimize workflows (Fig. 1).

Figure 1. DISPENDIX’s G.PREP NGS Automation system can run thousands of samples per day, automating multiple labor-intensive steps in next-generation sequencing to increase efficiency and accuracy.
The Need for Efficiency in Today’s Labs
The rise in popularity of experiments where sample numbers are high, workflows are complex, and high accuracy is required is leading to increasing pressure for labs to optimize their output. Manual workflows can slow down output: labor-intensive steps involving large amounts of pipetting can cause an experimental bottleneck as humans are limited in how quickly they can work. Furthermore, by trying to go faster errors become more likely, and injuries (such as repetitive strain injuries3) can occur.
Manual workflows also introduce variability into experiments whereby a batch effect - technical factors instead of the independent variable affecting the results - may occur and mask true biological differences4. Labs therefore need a solution for achieving faster turnaround, increased sample load, and consistent data quality.
How Automated Sample Preparation Systems Enhance Lab Efficiency
Automated sample preparation systems are a key method that labs are utilizing to improve lab efficiency by carrying out experiments quickly while maintaining accuracy.
Time-Saving Benefits
The use of automated sample preparation systems reduces the amount of time that researchers have to spend on laborious routine sample preparation. This allows them to focus their time on other, more technically challenging parts of the experiment, on data analysis, or on carrying out multiple experiments concurrently2. In addition, automated sample preparation systems increase throughput, enabling multiple samples to be processed simultaneously instead of sequentially and therefore reducing the time taken for sample preparation5.
Improved Accuracy and Precision
The labor- and time-intensive nature of sample preparation can result in human error, which increases with the number of steps and the total time taken to complete the process. Errors in sample preparation are common and can lead to incorrect data and erroneous conclusions6. Automated sample preparation systems remove the need for manual sample processing, thus decreasing human error, increasing the quality and precision of the results, and improving reproducibility7,8.
Cost Efficiency
The expense required to carry out high throughput experiments, such as next-generation sequencing, can limit the ability of labs to carry out these experiments9. Automated sample preparation systems can improve the cost efficiency of experiments by optimizing reagent use using microfluidic systems, this reduces the required volume of reagents and the dead volume, so reducing reagent costs10. For example, DISPENDIX has created technology where only 1 μL of dead volume is wasted per dispense.
Automated sample preparation systems can also reduce the costs associated with unnecessarily repeated experiments caused by human error or sample contamination11. Use DISPENDIX's G.PREP ROI calculator to see how much money automated sample preparation could save your lab.
Key Features of Automated Sample Preparation Systems
Versatility for Multiple Applications
Automated sample preparation systems have high versatility allowing their use in improving multiple systems. Key experimental processes with the potential for improvement through the use of automation include high-throughput, labor-intensive experiments with multiple-stepped workflows.
Next-generation sequencing is one such experiment where a complicated workflow must be carried out with high accuracy to produce high-quality results12. PCR is another experiment where the high numbers of samples and labor-intensive pipetting required benefits from the use of automation to remove human errors13. The I.DOT Liquid Handler produced has been optimized for high-throughput PCR plate preparation (Fig. 2).
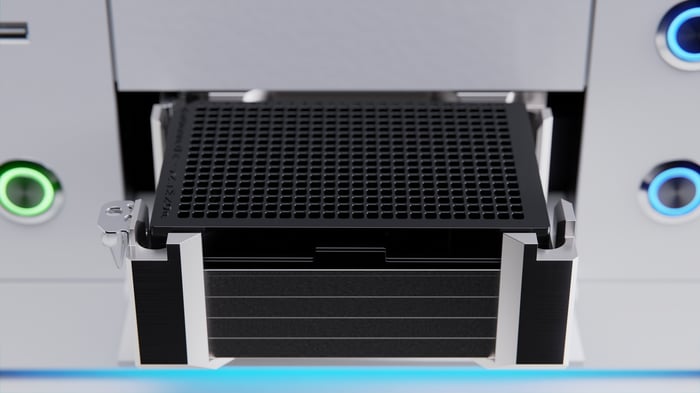
Figure 2. The I.DOT Liquid Handler can be used in PCR plate preparation to dilute samples and to add primers, probes, and mastermix directly to the samples accurately and without sample contamination.
High Throughput and Scalability
The scaling up of experiments for large-scale studies or clinical applications requires consistency. However, variability can arise through differences in sample preparation between researchers - such as manual pipetting variation14 - leading to batch effects and limited ability for inter-experiment comparison4. Automated sample preparation systems eliminate experiment-to-experiment differences in sample preparation, providing a path to scaling up systems for large-scale projects3.
Integration with Lab Automation
It is crucial for automated sample preparation systems to be compatible with existing equipment and to integrate well with other automated lab instruments in order to streamline workflows. The automation systems produced by DISPENDIX are designed not only to work effectively with other Dispendix technologies but also with a wide range of labware and lab equipment.
Conclusion
Automated sample preparation systems enable labs to meet the ever-increasing demands and pressures being put on them by complex, high-throughput experiments. By reducing the amount of time researchers spend on repetitive, laborious sample preparation, these systems allow researchers to use their time more effectively. Not only are they time-effective, automated sample preparation systems are also cost-effective, optimizing reagent use and reducing human-error-induced repeated experiments. Finally, these systems improve experiment accuracy, precision, and reproducibility, enabling the easy scaling up of systems without concerns about batch effects or compromising on quality.
Ready to accelerate your research?
Discover how DISPENDIX can enhance precision, efficiency, and reproducibility in your lab. Download the I.DOT brochure or the G.PREP brochure and take the next step in transforming your workflows!
References
- Pereira DA, Williams JA. Origin and evolution of high throughput screening. Br J Pharmacol. 2007;152(1):53-61. doi:10.1038/sj.bjp.0707373
- Socea JN, Stone VN, Qian X, Gibbs PL, Levinson KJ. Implementing laboratory automation for next-generation sequencing: benefits and challenges for library preparation. Front Public Health. 2023;11:1195581. doi:10.3389/fpubh.2023.1195581
- Holland I, Davies JA. Automation in the Life Science Research Laboratory. Front Bioeng Biotechnol. 2020;8. doi:10.3389/fbioe.2020.571777
- Leek JT, Scharpf RB, Bravo HC, et al. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet. 2010;11(10):733-739. doi:10.1038/nrg2825
- O’Neill MD. Increasing Throughput with Automated Liquid Handling. Genet Eng Biotechnol News. 2012;32(12):18-21. doi:10.1089/gen.32.12.06
- Hentz NG, Knaide TR. Effect of Liquid-Handling Accuracy on Assay Performance. SLAS Technol. 2014;19(2):153-162. doi:10.1177/2211068213504095
- Hess JF, Kohl TA, Kotrová M, et al. Library preparation for next generation sequencing: A review of automation strategies. Biotechnol Adv. 2020;41:107537. doi:10.1016/j.biotechadv.2020.107537
- Thaitrong N, Kim H, Renzi RF, Bartsch MS, Meagher RJ, Patel KD. Quality control of next-generation sequencing library through an integrative digital microfluidic platform. ELECTROPHORESIS. 2012;33(23):3506-3513. doi:10.1002/elps.201200441
- Grada A, Weinbrecht K. Next-Generation Sequencing: Methodology and Application. J Invest Dermatol. 2013;133(8):1-4. doi:10.1038/jid.2013.248
- Suckling L, McFarlane C, Sawyer C, et al. Miniaturisation of high-throughput plasmid DNA library preparation for next-generation sequencing using multifactorial optimisation. Synth Syst Biotechnol. 2019;4(1):57-66. doi:10.1016/j.synbio.2019.01.002
- Torres-Acosta MA, Lye GJ, Dikicioglu D. Automated liquid-handling operations for robust, resilient, and efficient bio-based laboratory practices. Biochem Eng J. 2022;188:108713. doi:10.1016/j.bej.2022.108713
- Cheng C, Fei Z, Xiao P. Methods to improve the accuracy of next-generation sequencing. Front Bioeng Biotechnol. 2023;11:982111. doi:10.3389/fbioe.2023.982111
- Zhu H, Zhang H, Xu Y, Laššáková S, Korabečná M, Neužil P. PCR Past, Present and Future. BioTechniques. 2020;69(4):317-325. doi:10.2144/btn-2020-0057
- Guan XL, Chang DPS, Mok ZX, Lee B. Assessing variations in manual pipetting: An under-investigated requirement of good laboratory practice. J Mass Spectrom Adv Clin Lab. 2023;30:25-29. doi:10.1016/j.jmsacl.2023.09.001