Next-generation sequencing (NGS) is a revolutionary DNA and RNA sequencing technique, overtaking Sanger sequencing as the most popular sequencing method. NGS has higher throughput, increased sensitivity, and is significantly more cost-effective than Sanger sequencing1. These factors enable its widespread use, leading to a huge impact on scientific research and healthcare, such as identifying disease-associated genetic mutations and tracing species evolution2.
Although NGS has many benefits, it is a complex technique. The workflow involves multiple processes that must be carried out with high accuracy in order to achieve high-quality results3.
Challenges in Traditional NGS Sample Prep
Labor-Intensive Process
Processes within the NGS workflow are notorious for being labor-intensive (Fig. 1), including repeated wash steps, precise pipetting, and time-sensitive steps. High levels of manual handling result in the increased likelihood of human errors. Errors can get amplified during the PCR stage and may result in PCR-exacerbated bias4. One mistake may be sufficient to ruin an entire day’s work, wasting time, money, and resources5.

Figure 1. NGS is a labor-intensive process requiring precise pipetting, which can result in a high risk of human error. (Source)
Time Constraints
The high-throughput abilities of NGS are limited by the speed of sample preparation. High-speed sample prep is difficult to achieve using manual processes, and human errors are exacerbated by attempting to work quickly. The requirement for hands-on work throughout sample preparation forces researchers to spend vast amounts of time on this process5. The use of manual processes therefore limits both the lab’s ability to perform large-scale experiments and the time available for carrying out innovative work.
Cross-Contamination Risks
Cross-contamination during NGS can lead to inaccurate results and data misinterpretation6. The risk of cross-contamination is high during several steps in the NGS workflow, including sample and library preparation7. Improper handling of samples is a substantial risk when processing samples manually, especially when attempting to process multiple samples simultaneously.
Scalability Issues
Consistency is key to scaling up NGS experiments for large studies or clinical applications, however, this is difficult to achieve with manual processing. Differences in sample preparation between researchers, such as variations in manual pipetting8, can lead to batch effects - where technical factors instead of the independent variable alter results. Batch effects can mask true biological differences and result in incorrect conclusions9.
How Automated Sample Prep Addresses These Challenges
Automated sample prep systems, such as Dispendix’s I.DOT, can be integrated into processes, including library prep, to address issues with traditional NGS sample prep methods (Fig. 2).
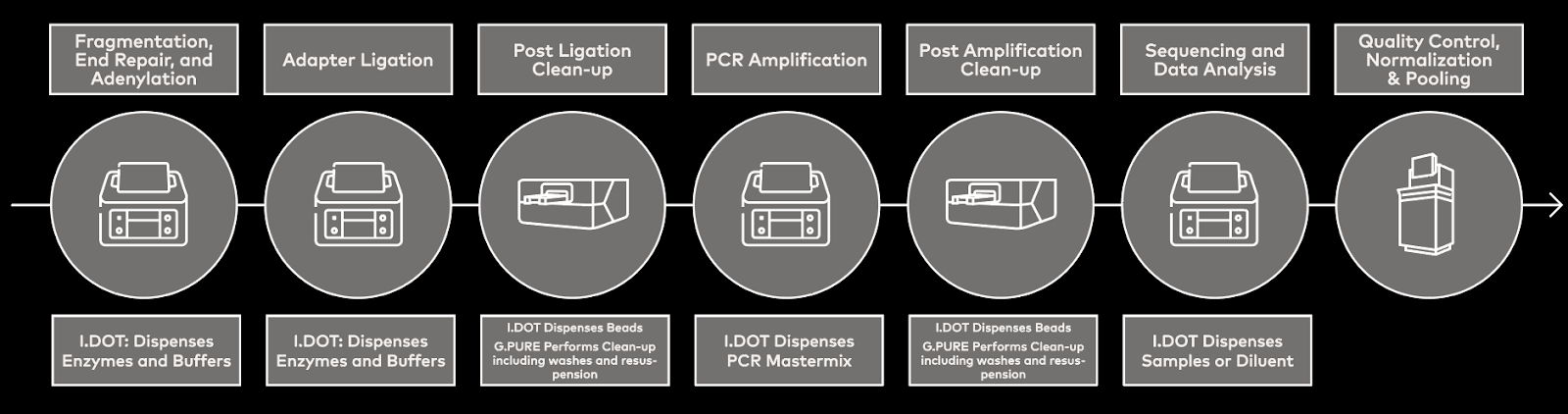
Figure 2. NGS workflow schematic demonstrating how automated sample prep using the I.DOT can be integrated into library prep.
Enhanced Accuracy and Precision
Human error in sample and library preparation methods is high, with inaccuracy and variability leading to decreased precision and reproducibility. Automated sample prep removes the need for manual sample processing. The automation of processes essential to the NGS workflow improves the performance of sequencing techniques10 and consequently the quality and precision of the results5.
Time Efficiency
Automated sample prep significantly reduces the amount of time that it takes for sample and library prep. These parts of the workflow are often considered bottlenecks in the NGS process as further steps cannot be carried out until these are complete10,11. Therefore, reducing the amount of time that these steps take enables the whole process to be completed faster. In addition, automated sample prep allows researchers to use their time more efficiently, as the time previously spent laboriously pipetting can now be spent on other tasks5.
Reduced Risk of Contamination
The use of automated sample prep allows improved methods of combating contamination. Not only does automation reduce the probability of human handling errors, but it can also carry out consumable-free clean-ups for NGS workflows, thus reducing contaminants. Automated systems are closed, resulting in reduced human intervention and minimizing environmental exposure10.
Scalability and High-Throughput Compatibility
Automated sample prep provides a way to reduce the batch effect between NGS experiments as researcher-to-researcher differences in sample preparation are eliminated12. This allows the easy scaling up of systems for large-scale projects without concerns about comparison between experiments or compromising on quality.
Key benefits of Automated Sample Prep Solutions for NGS
Automated sample prep improves the reliability of NGS data by reducing human error and inter-user variation to improve accuracy and precision. The risk of contamination is also reduced, lowering the probability of result bias and further improving NGS data reliability4–6. The NGS workflow can be carried out faster and more efficiently through the use of automated sample prep. In addition, recent optimizations in automated systems allow complicated workflows to progress with minimal oversight, enabling researchers to use their time more efficiently5.
The cost-saving benefits of automated sample prep are also evident. High reproducibility and reduced errors contribute to decreasing the costs associated with unnecessary mistake-induced repeat experiments. The efficiency of automated sample prep reduces the costs of wasted resources. Automated systems can minimize the reagent waste associated with human error in addition to allowing experiments to be carried out with smaller volumes of reagents13. Costs associated with consumables, such as pipette tips, can also be minimized through the use of automated sample prep, as automation can reduce or eliminate the need for these consumables14.
Try Dispendix’s ROI calculator to see how much you could save.
Conclusion
NGS is a game-changing sequencing technology being utilized in many areas of science and healthcare. It enables fast, high-throughput, and accurate DNA and RNA sequencing, however, there are challenges associated with the complexity of the workflow. NGS is a time- and labor-intensive process with multiple steps where human errors and inaccuracy may arise. However, automated sample prep enables many of these challenges to be overcome and offers further benefits. Automation reduces the risk of human error and sample contamination to produce accurate, reproducible results in a time- and cost-efficient manner. Labs carrying out NGS should therefore strongly consider implementing automated sample prep into their work to optimize their output.
Ready to automate your NGS workflow?
Download our G.PREP NGS Automation brochure and take the first step towards faster, more accurate sequencing results. Get your copy now!
References
- Behjati S, Tarpey PS. What is next generation sequencing? Arch Dis Child Educ Pract Ed. 2013;98(6):236. doi:10.1136/archdischild-2013-304340
- Kulski J. Next Generation Sequencing: Advances, Applications and Challenges. BoD – Books on Demand; 2016.
- Cheng C, Fei Z, Xiao P. Methods to improve the accuracy of next-generation sequencing. Front Bioeng Biotechnol. 2023;11. doi:10.3389/fbioe.2023.982111
- Head SR, Komori HK, LaMere SA, et al. Library construction for next-generation sequencing: Overviews and challenges. BioTechniques. 2014;56(2):61. doi:10.2144/000114133
- Socea JN, Stone VN, Qian X, Gibbs PL, Levinson KJ. Implementing laboratory automation for next-generation sequencing: benefits and challenges for library preparation. Front Public Health. 2023;11:1195581. doi:10.3389/fpubh.2023.1195581
- Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12(1):87. doi:10.1186/s12915-014-0087-z
- Kotrova M, Trka J, Kneba M, Brüggemann M. Is Next-Generation Sequencing the way to go for Residual Disease Monitoring in Acute Lymphoblastic Leukemia? Mol Diagn Ther. 2017;21(5):481-492. doi:10.1007/s40291-017-0277-9
- Guan XL, Chang DPS, Mok ZX, Lee B. Assessing variations in manual pipetting: An under-investigated requirement of good laboratory practice. J Mass Spectrom Adv Clin Lab. 2023;30:25-29. doi:10.1016/j.jmsacl.2023.09.001
- Leek JT, Scharpf RB, Bravo HC, et al. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet. 2010;11(10):733-739. doi:10.1038/nrg2825
- Hess JF, Kohl TA, Kotrová M, et al. Library preparation for next generation sequencing: A review of automation strategies. Biotechnol Adv. 2020;41:107537. doi:10.1016/j.biotechadv.2020.107537
- Syed F, Grunenwald H, Caruccio N. Optimized library preparation method for next-generation sequencing. Nat Methods. 2009;6(10):i-ii. doi:10.1038/nmeth.f.269
- Holland I, Davies JA. Automation in the Life Science Research Laboratory. Front Bioeng Biotechnol. 2020;8. doi:10.3389/fbioe.2020.571777
- Suckling L, McFarlane C, Sawyer C, et al. Miniaturisation of high-throughput plasmid DNA library preparation for next-generation sequencing using multifactorial optimisation. Synth Syst Biotechnol. 2019;4(1):57. doi:10.1016/j.synbio.2019.01.002
- Alves J, Sargison FA, Stawarz H, et al. A case report: insights into reducing plastic waste in a microbiology laboratory. Access Microbiol. 2021;3(3):000173. doi:10.1099/acmi.0.000173